
[MA149]Pages 30-58
(It may take a while to download but I think it is
worthy - John A. Keslick, Jr.) A word from the webmaster - Within
this article the word nutrient is misused at times where the true meaning is
essential elements. See my dictionary at
www.treedictionary.com and look up
nutrient as well as essential element. The reason for the latter is to
reduce misunderstanding of terms to better understand the message. John A. Keslick, Jr.
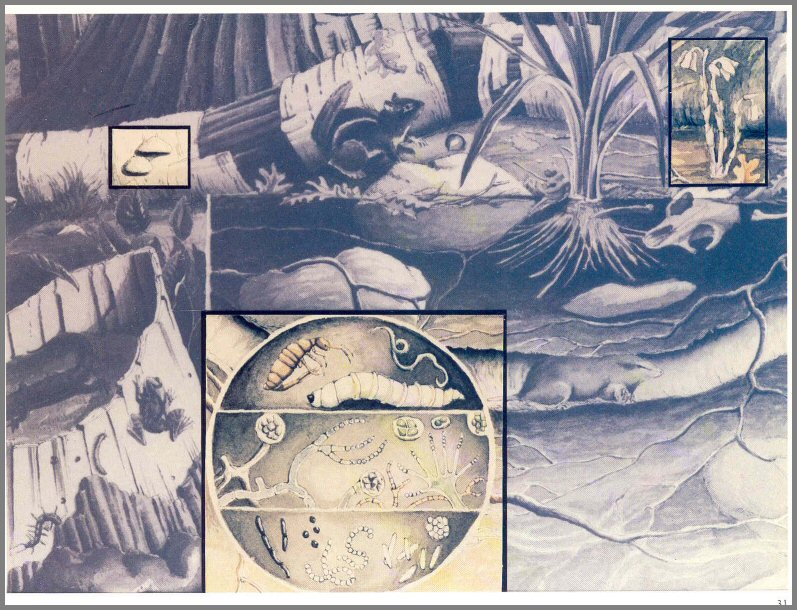
Figure 10. - Saprotrophs of the pond.

Page 30
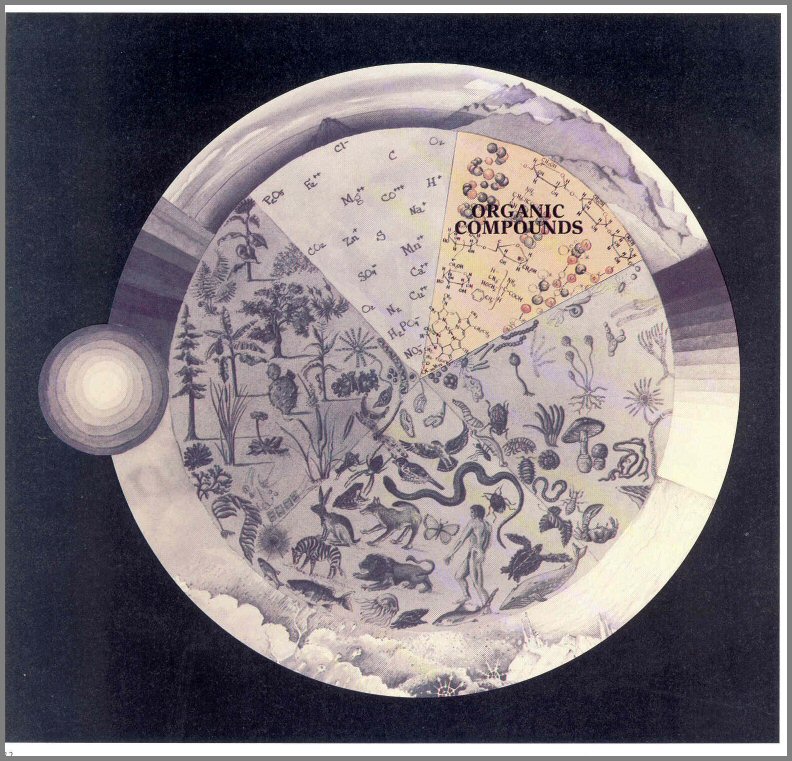
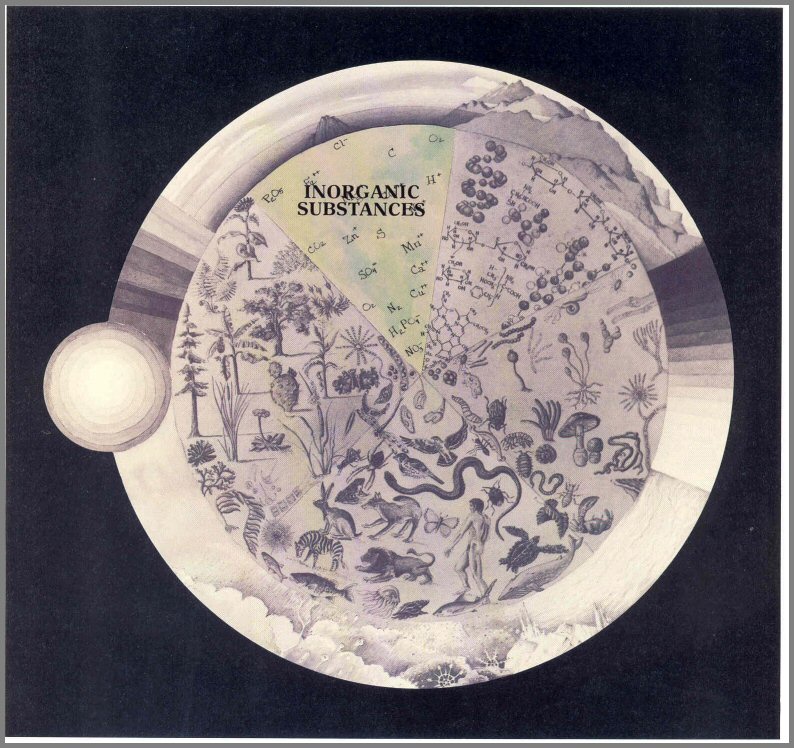
The activities of the biotic Players suggest that they are intimately interrelated with their nonliving environment. As we stated earlier, the abiotic components of the environment are also Players of the Game. We can divide them into three categories: Organic Compounds, Inorganic Substances, and the components of the Environmental Climate.
Figure 11. - Some forest Saprotrophs
Page 31
Page 32
ORGANIC SUBSTANCES
The Organic Compounds include carbohydrates, proteins, fats, and other compounds of plants and animals. Some of these compounds are easily digested by many kinds of organisms; some have molecules that resist breakdown by all but a few organisms with specially adapted enzyme systems.
Page 33
Page 34
INORGANIC SUBSTANCES
Primarily through the action of bacteria, the Organic Compounds are reduced to Inorganic Substances-elements such as phosphorus, nitrogen, and sulfur that can be recycled through the ecosystem beginning with the plant Producers. Sometimes these inorganic elements cannot be used directly, but must be recombined (again by the actions of special groups of bacteria) into other molecules. Thus plants use phosphorus as phosphates (H2 P04), nitrogen as nitrates (N03), and sulfur as sulfates (S04).
So the organisms in a natural ecosystem, interdependent because of food chain energy relationships, also depend on one another because they require growth substances that can be supplied only through the interactions of other organisms and the abiotic environment. The process by which essential materials are cycled between the living and nonliving Players is called biogeochemical cycling (see page 53). Nonessential and harmful materials also cycle through the biosphere.
Page 35

Page 36
ENVIRONMENTAL CLIMATE
In our pond ecosystem, the reproductive rate of the algal Producers and, therefore, of most of the other organisms, is strongly influenced by the temperature and chemical composition of the water, the depth of the water. The amount of shade from overhanging and floating vegetation, and by the general location and topography of the pond.
Similarly, in the forest, the species of plants that occur, how well they grow. and the types and numbers of animals they support directly reflect the nature of the soil, the annual distribution of rainfall, the length of the growing season, and many other factors of the physical nonliving environment.
IN YOUR ENVIRONMENTAL ARENA
What factors of the environmental climate are especially important influences on plants and animals in your area?
Page 37
III RULES OF THE GAME
The Rules that govern or regulate the Game of the Environment are
broad ecological principles applicable to all ecosystems. There are many
of these principles or biological Rules, but of paramount importance arc those
that relate to:
One-way flow of energy through a system.
Cycling of essential elements within
system.
Page 38
ENERGY FLOW RULES OF THE GAME
We on earth are entirely dependent on the sun for our energy-and we are but a way-station in the relentless path of energy's one-way flow from the sun to outer space. It is only because the green plant Producers have the fantastic and unique ability to use this light energy to convert carbon dioxide and water into sugars that we and all other life can exist. The constant rain of radiation energy on the earth is balanced by a nearly equal amount of heat energy that is returned from the earth to space. It is in the tissues-the protoplasm-of living and dead organisms that energy can be stored for awhile on earth as potential food energy for the Consumer Players, or for use by people as fuel.
First energy rule
Energy can neither be created nor destroyed, only converted from one form to another. This fact is the First Energy Rule; it is known as the First Law of Thermodynamics.
Page 39
Photosynthesis and respiration
Plants accomplish their life-giving function by photosynthesis. This remarkable example of the First Energy Rule occurs when the green chlorophyll of leaves converts carbon dioxide, water, and light energy into sugars and oxygen. A general equation for this process, the most important one in the world, is:

This, then, is how the sun's energy is converted into
potential food energy for growth. But unless the substances thus formed
are used, no growth can occur.
The process of breaking down the food produced by
photosynthesis, called respiration, is the way that all higher organisms
obtain their energy. Complete aerobic respiration (in the presence
of oxygen) yields carbon dioxide, water, and cell material; if the process is
incomplete, Organic Compounds that still contain energy remain. This
energy may be used later by other organisms.
Each year on earth, about 100 billion tons of organic matter
are produced through photosynthesis. And a large amount is oxidized back to
carbon dioxide and water through respiration (removal of O
Page 40

Page 41
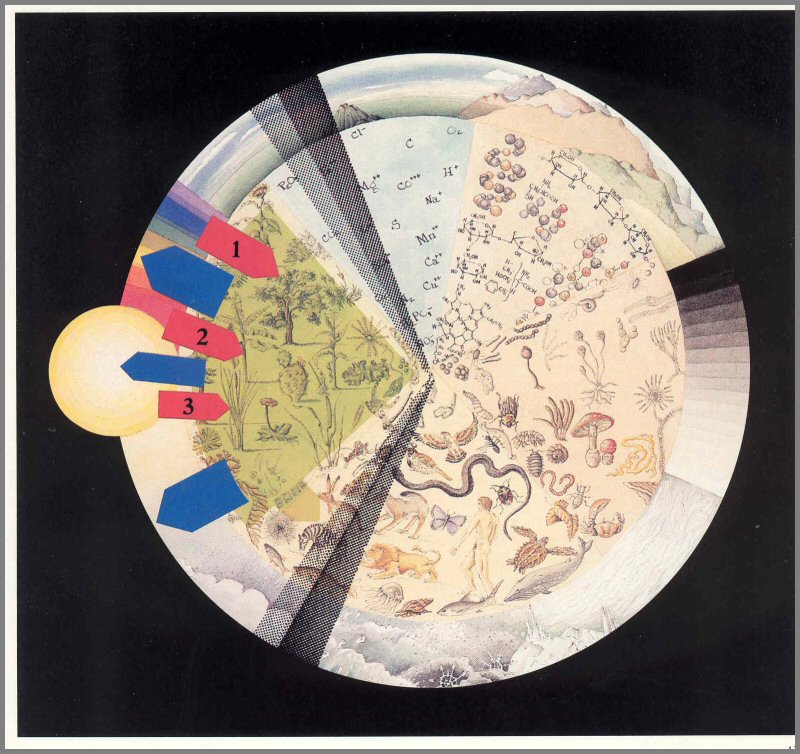
Solar radiation-sunlight-reaches the biosphere at a constant rate of 2 gram (g) calories per square centimeter (cm2) per minute, but only about two- thirds of this radiation reaches the ground. Of this light, 45 percent is visible, 45 percent is infrared, and 10 percent is ultraviolet. Plants use primarily the visible light from red and blue wavelengths in photosynthesis (food making), and they absorb some of the light from the long, far infrared wavelengths (fig. 12). And, while small amounts of green light and near infrared wave lengths are also absorbed and utilized by plants in important ways, most is reflected.
Page 42
Figure 12. - The sun's energy is converted by green plants to food energy. Red arrows: Light energy absorbed and utilized by plants. This includes blue and small amounts of green (1), red and small quantities of near infrared (2), and some far red (3) wavelengths. Green outline and arrow: net potential food energy produced through photosynthesis. Blue arrows: Energy reflected as light or heat into space.
Page 43
One-way flow of energy
The food chains shown in figures 8 and 9 point out some pathways along which masses of food energy pass through the ecosystem. What is not shown is the amount of food energy required to sustain one or all of the individuals at a trophic level; nor do these figures show the amount of heat energy that, generated in the maintenance of each organism, is radiated to space.
The different-size arrows in figure 13 represent the relative amount of food energy used for growth and maintenance (respiration), or that is stored by Consumers at each trophic level for use at the next higher level, or that is dispersed to space as heat.
Second energy rule
At each step in the flow of energy from one organism to another-from one trophic level to another-a great amount of potential chemical energy (food) is converted or degraded to heat to enable a small part to be reestablished as potential chemical energy (new protoplasm) in order to maintain the life of that second organism. The natural tendency, therefore, is for all light and chemical energy to eventually be degraded to and distributed evenly as heat energy. This transformation of energy from a concentrated state to a dispersed state comprises the Second Rule; it is known as the Second Law of Thermodynamics.
--- --- --- --- --- --- ---
IN YOUR ENVIRONMENTAL ARENA
Draw an energy flow diagram for an ecosystem near you. This could be your yard, a park, a garden, or a vacant lot. Include as many food levels as possible.
Page 44
Figure 13. - The one-way flow of energy through the ecosystem. Energy enters the system as light (red arrows), is transferred within the system as chemical food (green mass and arrows), and exits the system as heat (blue arrows). It does not recycle.
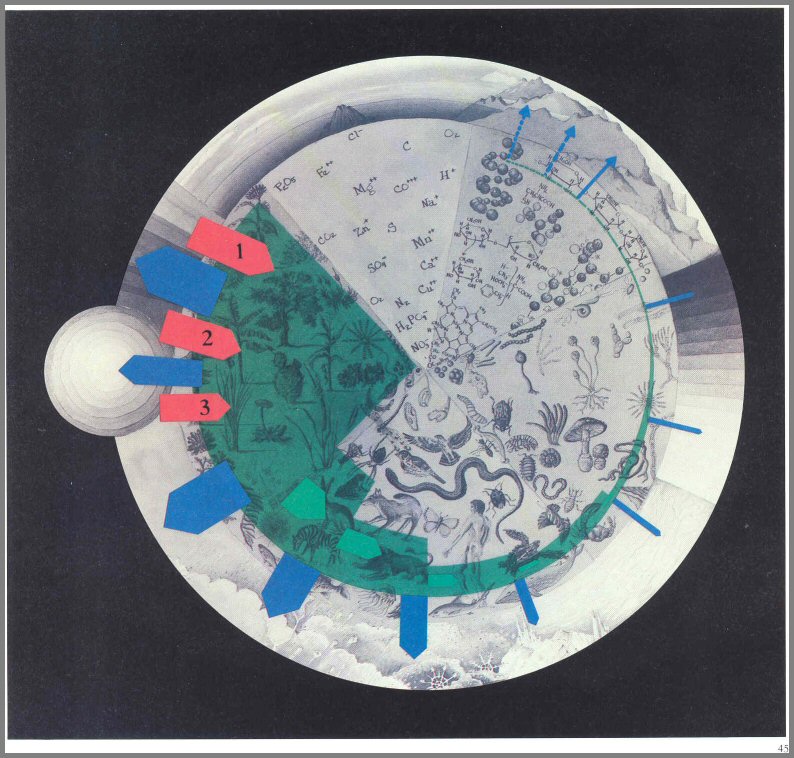
Page 45

Page 46-47
Figure 14.- The path of energy flow through a prairie ecosystem. Red arrow shows sunlight energy coming in; green arrows, food energy being passed from plants to animals; and blue arrows, heat energy being dispersed to space.
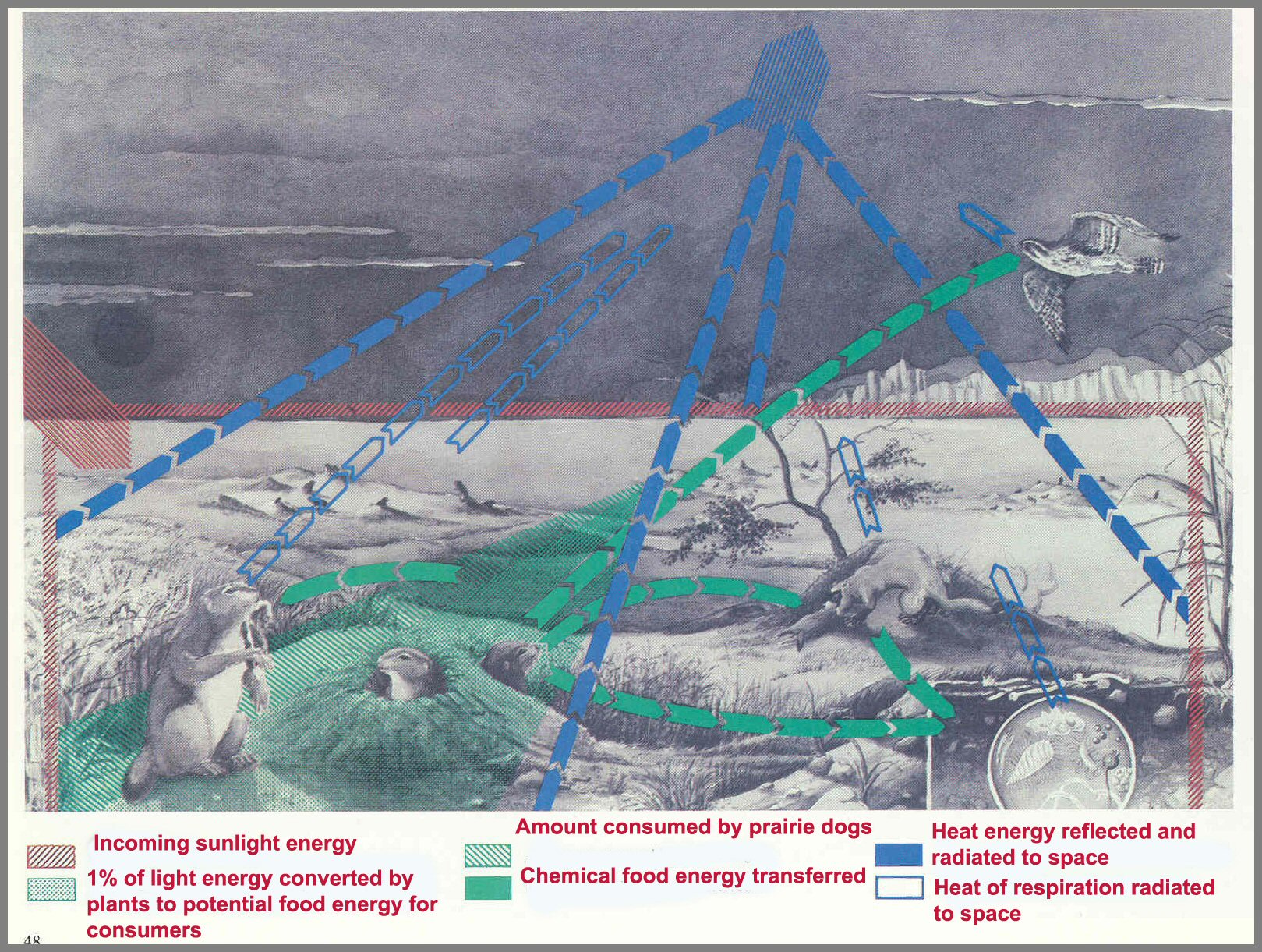
Page 48
An example of energy flow through a prairie ecosystem is shown in figure 14. In this Arena, light energy on prairie grasses (prairie cordgrass and wedgegrass) is shown by the red arrow. The bulk of light energy is reflected or is reradiated to space as heat (blue arrow). The plant Producers use about 1 percent of the light energy (wedge of 1 percent) to manufacture protoplasm and cell material and for respiration. Only a portion of the energy used by the Producer grasses is converted to potential food energy for use by the Consumer prairie dogs (green arrow), and only a portion of that small amount is actually consumed.
Much of the food energy consumed by the prairie dog is used to keep the animal warm (respiration) and is dispersed to space as heat. The small amount that is converted into prairie-dog tissues is available to its Consumers - primarily the black-footed ferret and, occasionally, the prairie falcon. These animals, in turn, convert most of this food energy to heat energy in respiration, but a small portion is stored in their new tissues. If there are no predators for these animals (if they are at the top of their food chain), their dead bodies (and those uneaten bodies at lower trophic levels) along with the unused portions of the prairie dogs (for example, bones and feces) are decomposed by the decomposer Saprotrophs. The respiration of the decomposer Players ultimately transfers to space, as heat energy, the last vestiges of the energy that entered the system as sunlight.
Page 49
Figure 15. - Ecological pyramids of numbers, biomass, and energy for a forest, a shallow pond, and an "old field. "

Page 50
Trophic level energy relationships
The relationships between trophic levels are of vital interest. How much acreage of a particular forage species must be grown to sustain a certain number of animals; how many parasites must be released to control a noxious insect; how many predators are necessary to maintain rodent populations at levels that will prevent unacceptable grain crop losses; or conversely, how many prey species are required to support viable populations of predators? These are some of the questions of trophic level relationships.
Ecological pyramids
One way of showing these relationships is by using
ecological pyramids. In figure 15, the shape of hypothetical pyramids
of numbers (individual organisms), of biomass (total mass (weight)
of organisms per unit area), and of energy (flow from one trophic level
to the next) are compared for a forest, a shallow pond, and an "old field."
The numbers pyramid is probably the least meaningful.
Knowing, for example, the tremendous numbers of daphnia (water fleas) required
to support one fish provides a dramatic picture; but this method is of little
use in comparing the pond to a different ecosystem because all individuals are
treated equally, regardless of size. It is difficult to compare numbers of
algal cells (pond) to grass plants (old field) or to trees (forest) (fig. 15).
A somewhat better comparison can be made with a biomass
pyramid. This pyramid provides a rough picture of the food chain
relationships for the entire ecosystem, especially when the sizes of the
organisms in successive trophic levels are similar. But Consumers may
outweigh the Producers; in lakes and oceans in winter, for example, small,
short-lived phytoplankton Producers may be outweighed by the larger,
longer-lived zooplankton Consumers. So the biomass pyramid of the ocean,
like the numbers pyramid of the forest, may be inverted.
The energy pyramid provides the best overview of trophic
level relationships. These pyramids show the rates at which food energy
passes through the food chain and allow comparisons of the same trophic levels
of different ecosystems and of the relative importance of individual groups
within a trophic level. In our example in figure 15, the amounts of food
energy available for the next trophic levels in the three ecosystems are of the
same "scale." These pyramids will always be right side up because of the
Second Energy Rule.
Page 51
Figure 16. - The cyclic flow of nutrients within an ecosystem. The arrows show the paths of nutrient flow between the living and non- living players.
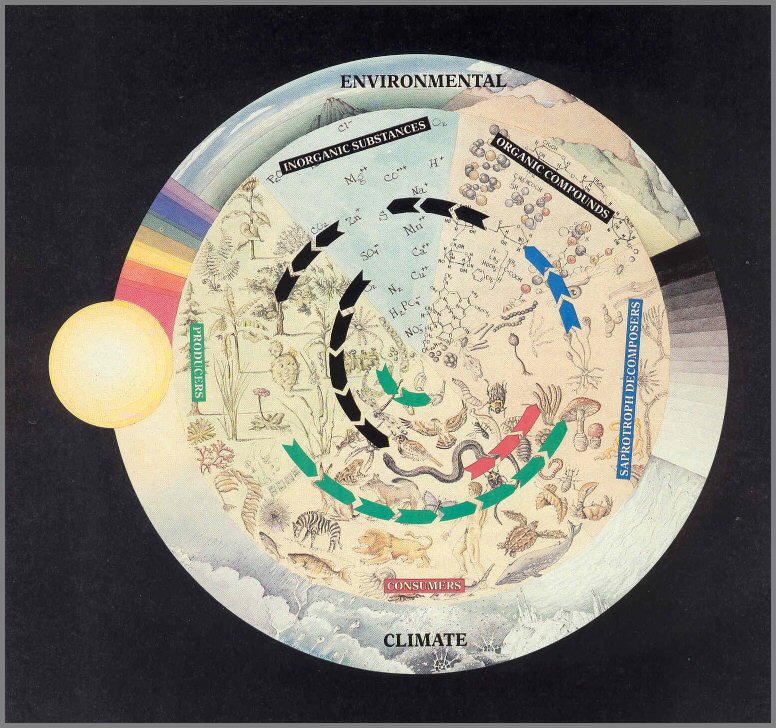
Page 52
MATERIAL CYCLING RULES OF THE GAME
We stated earlier that biogeochemistry is the study of
the exchange of materials between the living and nonliving Players.
Biogeochemical cycles show the pathways for the circulation of nutrients
essential for life (from environment to organisms to environment). The
movement of nutrients through an ecosystem occurs as energy is transformed from
one state to another, but unlike energy, whose flow is one way, the flow of
nutrients is cyclic (fig. 16).
The cycling of nutrients is made possible by the Saprotroph
Players, the macroconsumers and microconsumers that break down
dead tissues of Producers and Consumers. Thus essential elements
for growth such as nitrogen, phosphorus, sulfur, carbon, and calcium from the
decomposition process are absorbed and used by the green plants, by Consumers at
several trophic levels, and again by the plants.
Cycle pools
Each nutrient cycle has two compartments or "pools." The reservoir
pool, the largest, can be immediately unavailable to Players because of its
location or the chemical form of the nutrient. The smaller active pool
is available to organisms and is cycled or exchanged between them and their
immediate environment.
Types of cycles
When the reservoir pool is located in the earth's crust, the nutrient cycle is
termed sedimentary. When this pool is in the atmosphere or
hydrosphere, the nutrient cycle is a gaseous one.
The biogeochemical cycles in figures 17 through 28 are
examples of how water and a few of life's essential nutrients move within
ecosystems. It should be recognized that there also are cycles of many
materials that are harmful to life.
The effects of some of these latter cycles are discussed on pages 127 through
137.
Page 53
Page 54 -55
Figure 17. - The primary pathways and processes of the water cycle. Passive movements of liquid water (condensation, precipitation, percolation, and overground transport) are shown by green arrows, active uptake by plants and animals (absorption and ingestion) are shown by red arrows, and the return to the atmosphere (evaporation, transpiration, and respiration) are shown with blue arrows.
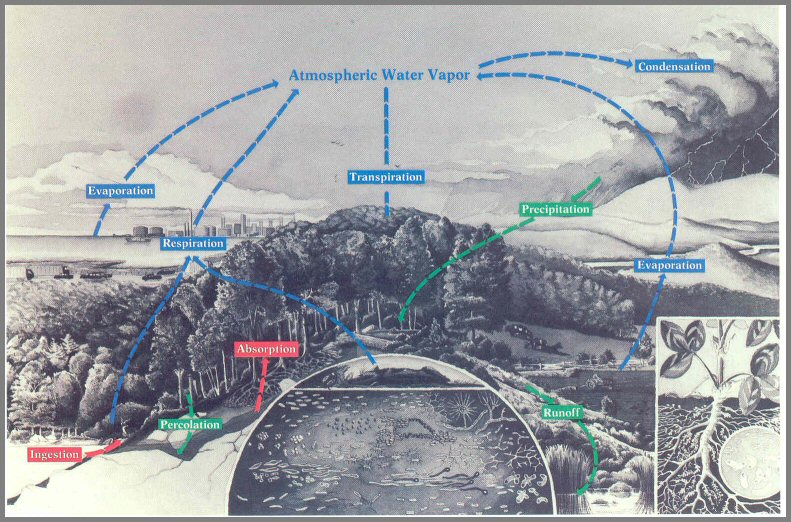
Page 56
SOME IMPORTANT BIOGEOCHEMICAL CYCLES
The water cycle
Water is by far the most abundant and most important single substance in the
biosphere. Fresh water is present as water vapor in the atmosphere; as a
liquid in the atmosphere, on land, and in water bodies; and as solid ice in the
polar caps and glaciers. But about 97 percent of all water is the liquid
salt water of the oceans and seas.
Water is a major geological force. Fracturing and
pulverizing rock by its unique property of expanding upon freezing, water erodes
and transports sediments and dissolved substances from high elevations to low
ones, from one location to another. It is the primary force in leveling
mountains and in filling valleys and lowlands. Because water stores heat
energy better than any other liquid, it can transport heat energy great
distances. Examples of this important characteristic include transporting
heat across the Atlantic by the Gulf Stream current and the use of water to
carry heat from nuclear reactors. This same property is a powerful factor
in regulating climate-especially near coastal areas.
Water is the medium of all life processes. It
transports nutrients as it flows through soil and through living matter.
Water also removes and dilutes many natural and artificial wastes.
Like all biogeochemical cycles, the water cycle (fig. 17) is
powered by energy from the sun. Water vapor enters the atmosphere through
evaporation from bodies of water and from soil and through
transpiration from the leaves of vegetation. This water vapor in the
cool reaches of the atmosphere condenses to form clouds
(condensation). When water droplets become heavy, they leave the
atmosphere and return to earth as rain or snow
---- ---- ---- ---- ----
IN YOUR ENVIRONMENTAL ARENA
Draw a diagram showing
how you fit into the water cycle.
List the sources of water in your
community.
How is water used in your community?
Is the water treated before it can be
used? If so, how? Why is it treated?
What can you do to help conserve water?
Page 57
The carbon cycle
We usually think of the carbon cycle (fig. 18), one of the most important to
man, as the carbon dioxide cycle because most of the carbon is available as
carbon dioxide in the atmosphere, as dissolved carbon dioxide in oceans, or as
"stored" carbonates. Excess carbon dioxide in the atmosphere dissolves in
the oceans and atmospheric deficits are replaced by carbon dioxide from the
oceans. Thus the oceans partially regulate this cycle (fig. 18, blue
arrow).
Green plants use carbon dioxide from air or water to make
carbohydrates and fats (production by photosynthesis) as was
mentioned on page 40. These energy-rich compounds are passed on to
Consumers who cannot synthesize their own Organic Compounds directly from carbon
dioxide and water. As plants and animals respire (respiration) and,
finally, when their bodies decompose (decomposition) carbon dioxide is
returned to the atmosphere. When production exceeds decomposition, organic
matter accumulates and becomes incorporated in the earth's crust.
Today, people are materially affecting the carbon cycle by
their activities that require the tremendous combustion of fossil fuels
and the increased oxidation of organic matter in soil through agriculture
and the draining of wetlands. Each year, these activities inject into the
atmosphere billions of tons of carbon dioxide.
Over the last half century, the carbon dioxide content in the
atmosphere has increased by about 13 percent; it has been predicted that
atmospheric carbon dioxide will increase by an additional 25 percent by the year
2000. These increases may significantly affect our global climate.
In the processes shown in figure 18, carbon is always
associated with oxygen, so the carbon cycle also can be considered an oxygen
cycle. Oxygen produced during photosynthesis is consumed by animals
and plants in respiration and it is used in the processes of
decomposition, combustion, and oxidation.
Page 58